 Thin layer chromatography is probably the easiest way how to perform chromatographic separation. At least you do not need any instrument. In thin layer chromatography (TLC) the solvent flows through the stationary phase which covers the thin plate. One part of plate is submerged into the mobile phase which travel across the plate using capillary forces.
Thin layer chromatography is probably the easiest way how to perform chromatographic separation. At least you do not need any instrument. In thin layer chromatography (TLC) the solvent flows through the stationary phase which covers the thin plate. One part of plate is submerged into the mobile phase which travel across the plate using capillary forces.
The sample spots drift towards the second end of the plate according their interaction with the stationary phases. Some of them travel faster then other, hence resulting separation occurs.
One part of thin layer chromatography uses paper as a stationary phase and is accordingly called paper chromatography.
Paper chromatography
Paper chromatography is probably the simplest form of any kind of chromatography and (probably therefore) is widely used. Most people meet chromatography in its paper version at school.
The fibres of paper (cellulose) may be used either directly as a stationary phase or can provide support for liquid stationary phase (for example water).
Sample preparation and separation development
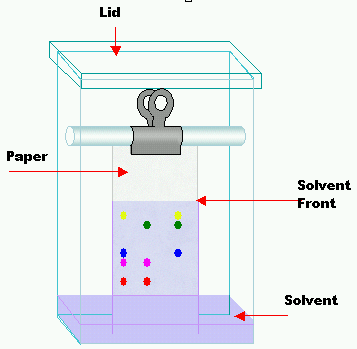
First, you need to draw a thin pencil line at a distance of a 1 – 2 cm from the bottom of the paper. This line servers as a starting reference for the calculation of Rf values. Why pencil? Because ink contains soluble pigments that will separate when the mobile phase is running over the paper.
The solution of sample is then introduced on the line at the paper. It is important to make sample spots both as concentrated as possible and as small as possible. You can add several very small spot additions on the top of previously dried sample. Very useful for sample introduction are glass capillaries. The smaller the better.
The paper with samples should be hung from a support to allow the bottom end of the paper to be immersed into a chromatography tank – in this case, the mobile phase then travel up the paper forced by capillary action.
Alternatively, the paper can be curl into the form of tube and secured by paper clip. This tube can then stay vertically inside the tank.
In both cases, be careful and immerse paper cautiously. The mobile phase should be about 1 cm below the line with the samples. Otherwise, samples can be washed out by the mobile phase. And you don’t want it, don’t you?
Finally, lid should be placed on the tank, so the atmosphere surrounding the paper is saturated with the mobile phase’s vapor.
Before the solvent reaches the end of the paper, second pencil line is to mark the distance traveled by the mobile phase. Paper can be then removed and dried.
Identification and detection
The retention factor, Rf , which characterizes the retention of each compound is than calculated as the ratio between the distance of the spot from the beginning and the distance of the solvent front:
Rf = distance traveled by the spot / distance traveled by solvent
The Rf of compound should be the same both in case of separation of complex mixture and in case of individual compound traveling through the paper. Thus, it is possible to identify spots by their Rf values if the individual compounds traveled together with the analyzed mixture.
Retention factor values depend highly on experimental conditions and, if we want to identify spots in the mixture, we should always run mixture together with individual compounds.
In case of colored compounds it is very easy to see where the spots have traveled. A number of dyes and inks can be separated into their individual components. In this way, we might find out how many different pigments are in blue, black inks, or food dyes.
You might check the composition of ink even at home with very easy paper chromatography experiment.
Non-colored components can be detected by post-analysis derivatization. For example, amino acids can be analyzed with separated in the mobile phase formed from a 4 : 1 : 5 mixture of 1-butanol, glacial acetic acid, and water. The paper can be then sprayed with ninhydrin, which change the color of amino acids to purple and allows the identification of spots on the paper.
Thin layer chromatography
Thin-layer chromatography (TLC) is very similar to paper chromatography. The advantage of TLC is that offers better separation with higher reproducibility.
TLC uses as stationary phase solids such as alumina or silica immobilized on a glass or polymer plate. Alumina is is very polar and separation between the stationary and mobile phase may involve adsorption, partition, and/or ion exchange process. The mobile phase may be water, aqueous ammonia solution, mixtures such as an alcohol/water/acetic acid solution or other organic solvents.
Thin layer chromatography plates are developed in the same way as a paper in paper chromatography. Pencil lines are drawn above the mobile phase level and plates are placed upright in a chromatographic tank. Special care should be taken so the plates are not scratched. Otherwise it might impair the separation.
Alumina as a stationary phase is very often coated with fluorescent material which helps visualize compounds as they are separated on the plate. The compounds quenches the fluorescence, so they are visible as a black spots under UV light.
Detection in thin layer chromatography
Again, the retention factors can be calculated and applied for the component identification. TLC plates are often treated with reagents such as iodine or derivatizing agents to visualize compounds that cannot be seen by naked eye.
Thin layer chromatography is normally used for a qualitative analysis of non-volatile compounds such as pharmaceuticals or dyes. In organic chemistry, TLC is very often used to determine if synthetic samples contain impurities (single spot – pure compound, several spots – impurities).
Quantification in thin layer chromatography is difficult, since it is hard to quantitatively deposit known quantities of mixture on the plate.
to be continued …